I-131 Sodium Iodide
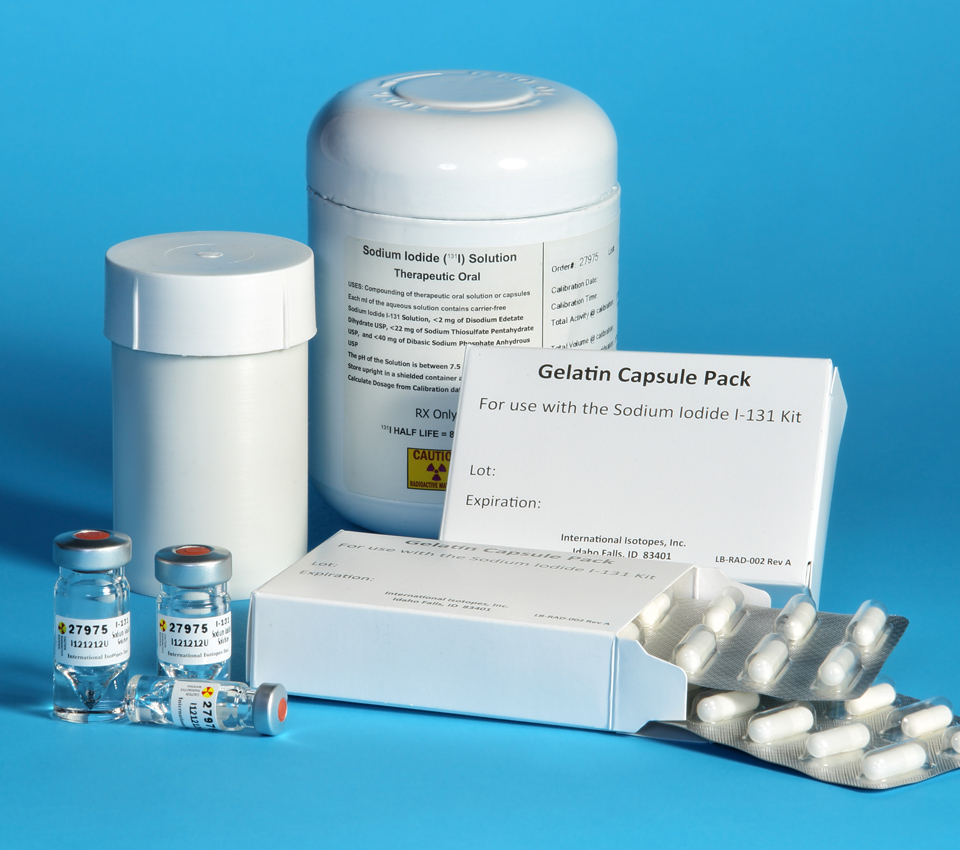
Sodium Iodide I-131 Kit for the preparation of Sodium Iodide I-131 Capsules and Solution USP Therapeutic – Oral
International Isotopes Inc. (OTCQB: INIS) is proud to be the only FDA approved Sodium Iodide I-131 drug product manufactured in the United States. Sodium Iodide I-131 is a radioactive therapeutic agent indicated for the treatment of hyperthyroidism and selected cases of carcinoma of the thyroid.
Solutions are custom made to order in any size increments from 250 to 2250 mCi/mL for the radiopharmaceutical grade. Our Sodium Iodide I-131 kits offer you a variety of vial sizes and a range of activities.
International Isotopes Inc. takes pride in providing you with exceptional and responsive customer service throughout the ordering process. We work to accommodate your varied order needs and delivery schedules. Our goal is to reliably provide you with high quality GMP product on a schedule to best meet your needs.
• Fixed concentration for Radiopharmaceutical product is 1000 mCi/mL.
• Radiopharmaceutical product has 14 day shelf life.
• Radiopharmaceutical product vial sizes are 1, 2, 3 mL V-Vials.
• Two days of pre-calibration included from time of shipment from INIS.
Manufacturing and Distribution of Sodium Iodide I-131 solution
All facets of the Sodium Iodide I-131 solution manufacturing and distribution are conducted in accordance with FDA 21 CFR 211, current good manufacturing practice (cGMP) for finished pharmaceuticals. You can rest assured that the International Isotopes Sodium Iodide I-131 solution’s quality, efficacy, and safety has been evaluated by the FDA.
Our dedication to quality at International Isotopes doesn’t stop at compliance to FDA regulations. We are also certified to ISO 9001 (current edition) for quality management systems. Compliance to the ISO standard provides a framework for managing quality that is acknowledged internationally.
The regulatory cGMP concepts and quality management system used to make prescription sodium Iodide I-131 solution are also applied to the manufacture of radiochemical sodium Iodide I-131 solution.
All orders of sodium Iodide I-131 solution, drug or radiochemical, are packaged and shipped in compliance with all US Department of Transportation (DOT) and Nuclear Regulatory Commission (NRC) requirements.
Sodium Iodide Orders/Questions
For ordering information or questions, use the form below or call us at 208.524.5300.