International Isotopes, Inc.
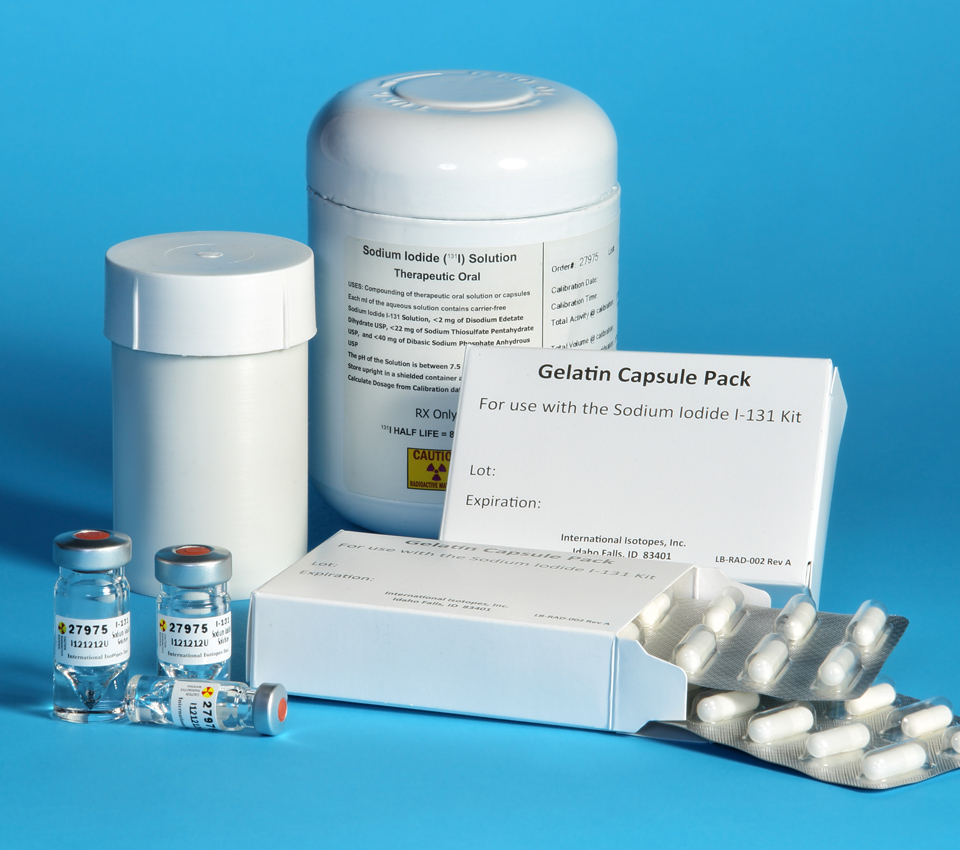
I-131 Sodium Iodide & Other Radiochemicals
International Isotopes Inc. (OTCQB: INIS) is proud to be the only FDA approved Sodium Iodide I-131 drug product manufactured in the United States.
Nuclear Medicine Calibration Standards
Calibration standards are used for a range of applications in nuclear pharmacy from testing of imaging machines to calibration of patient dose measurement devices.
Cobalt Sealed Source Products
Our cobalt products are used by a broad array of companies for radiation treatment of cancer and vascular deformities of the brain. Cobalt sources are also used for container security examinations at our sea ports and borders.
Calibration Sources & Radioactive Standards
We are the North American distributor for LEA sources for control and calibration of equipment in the fields of radiation protection and metrology.